In the realm of weight management and type 2 diabetes treatment, three injectable medications have garnered significant attention: Mounjaro, Wegovy, and Ozempic. Each offers unique benefits, mechanisms of action, and potential side effects. Understanding these distinctions is crucial in determining which medication aligns best with your health goals.
Indications and Approvals
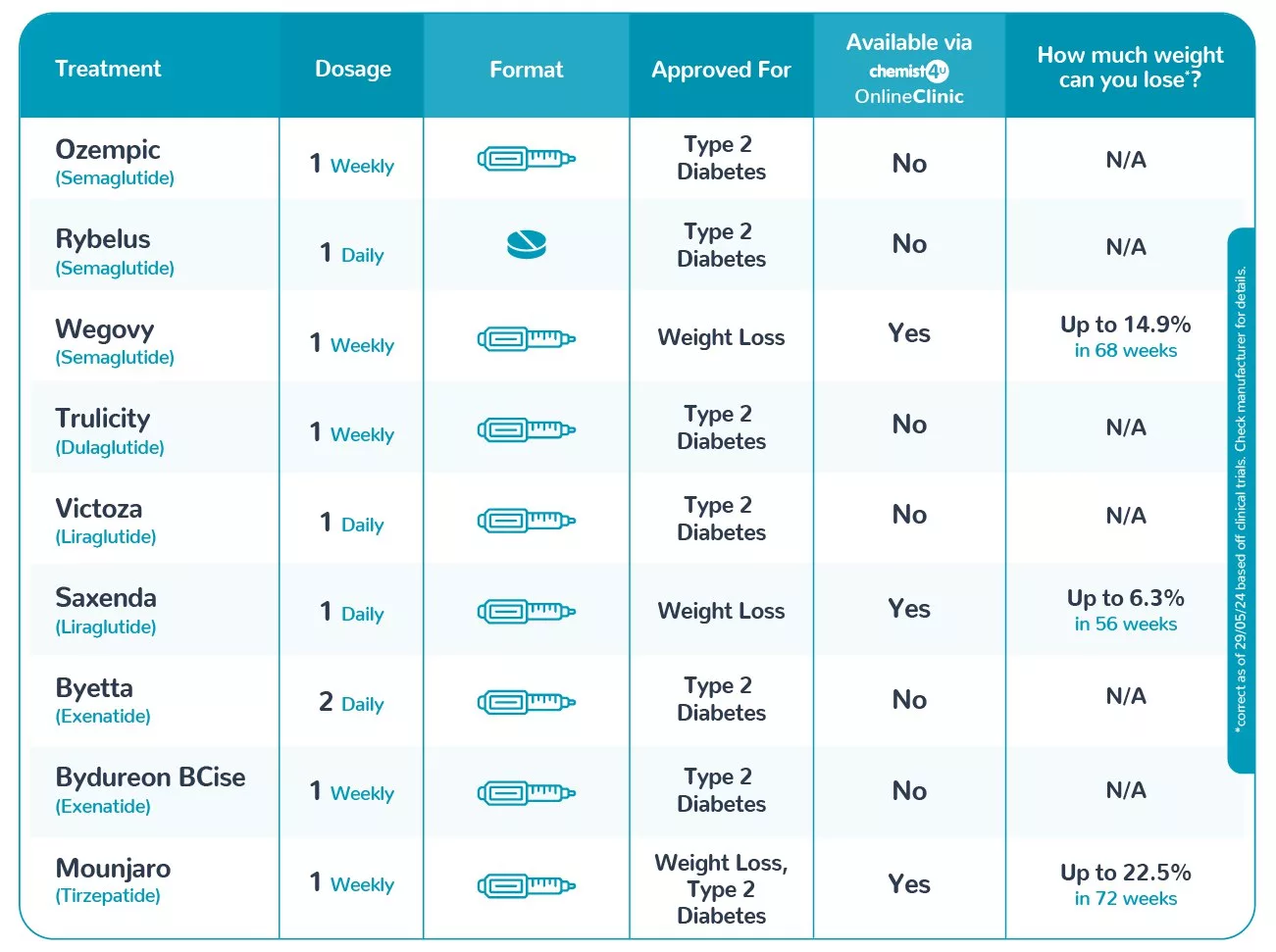
- Mounjaro: Primarily approved for type 2 diabetes management, Mounjaro (tirzepatide) is also authorized for weight loss in adults with a body mass index (BMI) of 35 kg/m² or higher, particularly when dietary interventions have not yielded desired results.
- Wegovy: Specifically approved for weight management, Wegovy (semaglutide) is indicated for adults under 65 years with a BMI of 35 or more. It functions by slowing digestion and enhancing feelings of fullness, leading to significant weight reduction.
- Ozempic: Designed for type 2 diabetes treatment, Ozempic (semaglutide) aids in blood sugar regulation. While not officially approved for weight loss, many patients experience secondary weight reduction during treatment.
Mechanisms of Action
- Mounjaro: Combines the effects of GLP-1 and GIP receptor agonists, enhancing insulin secretion, improving the body’s insulin response post-meals, and slowing gastric emptying. This dual action reduces appetite and promotes significant weight loss.
- Wegovy and Ozempic: Both utilize semaglutide, a GLP-1 receptor agonist. They increase insulin production in response to blood sugar levels and delay gastric emptying, promoting prolonged satiety and reduced food intake.
Dosage and Administration
- Mounjaro: Administered via weekly subcutaneous injections, with dosages adjusted based on individual patient needs and treatment response.
- Wegovy: Initiated at 0.25 mg per week, gradually increasing over 16 to 20 weeks to a maintenance dose of 2.4 mg weekly. This gradual escalation helps mitigate gastrointestinal side effects.
- Ozempic: Starts at 0.25 mg weekly, increasing to 0.5 mg or 1 mg as needed. For more stringent blood sugar control, doses up to 2 mg may be considered.
Efficacy in Weight Loss
- Mounjaro: Clinical studies have shown that patients can lose up to 20-25% of their initial body weight after 72 weeks of treatment.
- Wegovy: Patients may experience an average weight loss of 15-20% over 68 weeks, making it a potent option for obesity management.
- Ozempic: While primarily targeting blood sugar control, Ozempic users often see a moderate weight loss of approximately 5-10% of their body weight.
Potential Side Effects
- Mounjaro: Common side effects include nausea, vomiting, diarrhea, and reactions at the injection site. There’s also a rare risk of pancreatitis.
- Wegovy: Patients may experience gastrointestinal issues such as nausea, diarrhea, and abdominal discomfort. Due to higher dosing, these effects can be more pronounced.
- Ozempic: Similar to Wegovy, with potential for hypoglycemia, especially when combined with other antidiabetic medications.
Making the Right Choice
Selecting between Mounjaro, Wegovy, and Ozempic depends on individual health objectives:
- For significant weight loss: Wegovy may be the most suitable due to its targeted approval for weight management.
- For type 2 diabetes management with weight loss benefits: Mounjaro offers a dual mechanism that addresses both blood sugar control and substantial weight reduction.
- For primary blood sugar control with moderate weight loss: Ozempic serves as an effective treatment.
It’s essential to consult with a healthcare professional to determine the most appropriate medication based on your specific health profile and goals.
At chatgpt-pharma.com, we provide comprehensive information and support to help you make informed decisions about your health. Explore our resources to learn more about these medications and how they can assist you in achieving your health objectives.




Write a comment
You must be logged in to post a comment.